Last Updated: 4/19/22
The Compliance option in the Proposal Development document is used to indicate when circumstances exist that may require additional review. Those circumstances are:
- The inclusion of human subjects, whether sponsor or MSU initiated
- The inclusion of animals
- If international activities are taking place
- The need for additional space or renovations
- COI – Board of Trustee Review
- The inclusion of export-controlled information, if known at the time of proposal
WHO:
- College/Department Administrators
- Principal Investigators/Key Personnel
WHEN:
- Proposal includes one of the circumstances listed above
HOW:
- Click on the Compliance option within the Proposal Development document.
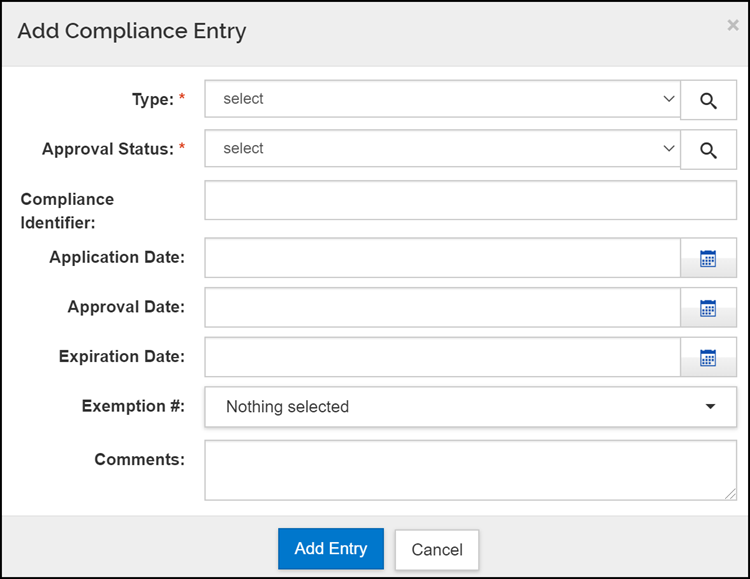
- Click on the Add compliance entry button at the top of the screen.

- Select the Type from the drop-down menu, this is a required field and must be completed.
- Select the Approval Status from the drop-down menu, this is also a required field.
- For Human Subjects and Animal Use, unless there is already an approved study/protocol, Pending should be selected as the Approval Status, even if the Investigator has not yet applied for approval. This is to fulfill federal form requirements.
- Enter the Compliance Identifier, if known. A Compliance Identifier is required if you have selected Human Subjects as your Type, with Approved as the Approval Status.
- Enter the Application Date if you have already submitted an IRB or IACUC study/protocol for review.
- Enter the Approval Date, if you have received an approval letter from the IRB or IACUC for a non-exempt study/protocol.
- Enter the Expiration Date, if you have received an approval letter from the IRB or IACUC for a non-exempt study/protocol.
- Select the Exemption # from the scroll down list when “Human Subjects” is selected for Type and the Approval Status is exempt. Please note the Exemption field is intended exclusively for system-to-system proposals and is related to OHRP regulations. You can select multiple exemption numbers from the list by holding down the control key when clicking on subsequent values.
- Additional details may be provided in the Comments field.
- Click the Add Entry button to add the item to the Compliance option.
- Select the Save or Save and Continue button at the bottom of the page.
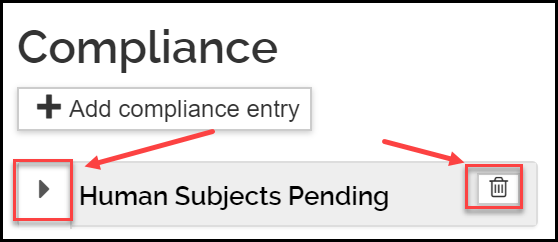
- Once added, to view or edit the information, click the triangle icon next to the entry. To delete the entry, click the trash icon.